
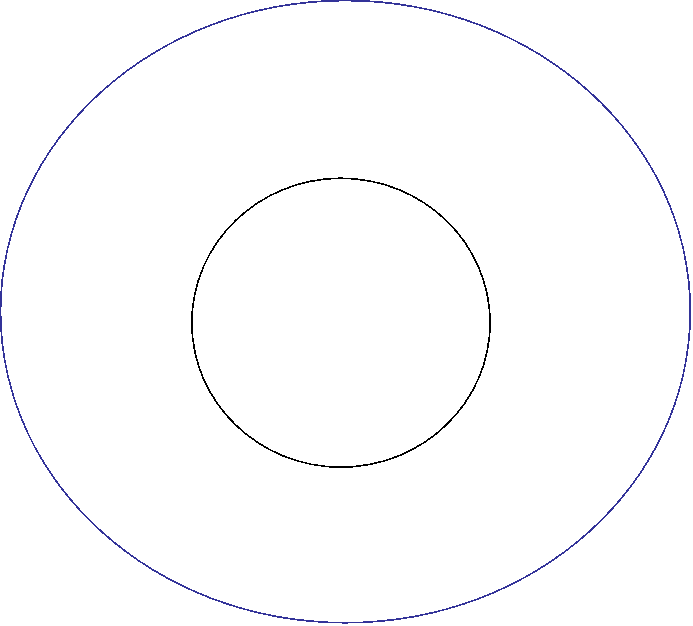
The Balmer Lines
n = 1
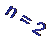
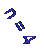
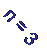


Ha
Hb
Hg
The only hydrogen lines in the visible wavelength range.
Transitions from 2nd to higher
levels of hydrogen
2nd to 3rd level = Ha (Balmer alpha line)
2nd to 4th level = Hb (Balmer beta line)
…
