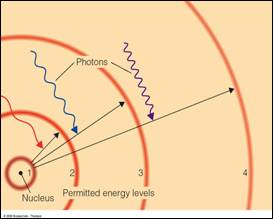
Atomic Transitions
•An electron can be kicked into a higher orbit when it absorbs a photon with exactly the right energy.
•All
other photons pass by the atom unabsorbed.
Eph = E4 – E1

Eph = E3 – E1

(Remember that Eph = h*f)

Wrong
energy
•The photon is absorbed, and the electron is in an excited state.
