18
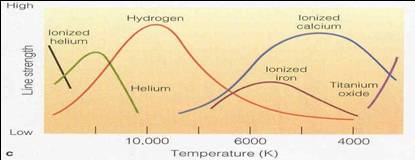
Sense larger T range using many atoms
•Different
atoms hold on to electrons with different force
–Use weakly held electrons to sense low
temperatures (Fe, Ca, TiO)
•TiO molecule is destroyed above 4000K
•Ca has lost 1 electron by ~5000K, but
still has others to give lines
–Use moderately held electrons to sense
middle temperatures (H)
•Below 6000 K most H electrons in lowest
state – can’t cause Balmer lines
•Above 15,000K most H electrons
completely lost (ionized)
–Use tightly held electrons to sense high
temperatures (He, ionized He)
•Below 10,000K most He electrons in ground
state – just like H, no visible absorption lines
•Above 15,000K most H has lost one
electron, but still has a second one to cause absorptions
From our text: Horizons, by Seeds