| Which levels will be occupied? |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
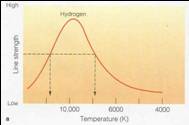
| • | The higher the temperature, the higher the typical level |
||||||||||||
| – | Collisions can knock electrons to higher levels, |
||||||||||||
| if moving atoms have enough kinetic energy |
|||||||||||||
| – | At T ~ 300 K (room
T) almost all H in ground state
(n=1) |
||||||||||||
| – | At T ~ 10,000 K many H are in first excited state (n=2) |
||||||||||||
| – | At T ~ 15,000 K many H are ionized |
||||||||||||
| • | Because you have highest n=2 population at ~10,000K |
||||||||||||
| you also have highest Balmer line strength there. |
|||||||||||||
| • | This gives us another way to estimate temperatures of stars |
||||||||||||