16
Hydrogen
Lines
•Energy absorbed/emitted depends on upper and
lower levels
•Higher energy levels are close together
•Above a certain energy, electron can
escape
(ionization)
•Series of lines named for bottom level
–To get absorption, lower level must be
occupied
•Depends upon temperature of atoms
–To get emission, upper level must be
occupied
•Can get down-ward cascade through many
levels

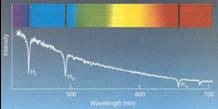
From our text: Horizons, by Seeds
n=1
n=2
n=3