8
Atoms –
Electron Configuration
•Molecules: Multiple
atoms sharing/exchanging electrons
(H2O, CH4)
•Ions:
Single atoms where one or more electrons have escaped (H+)
•Binding
energy: Energy needed to let electron
escape
•Permitted
“orbits” or energy levels
–By rules of quantum mechanics, only certain
“orbits” are allowed
–Ground
State:
Atom with electron in lowest energy orbit
–Excited
State:
Atom with at least one atom in a higher energy orbit
–Transition: As electron jumps from one energy
level orbit to another,
atom must release/absorb energy different, usually in form of light.
atom must release/absorb energy different, usually in form of light.
•Because only certain orbits are allowed, only certain
energy jumps are allowed, and
atoms can absorb or emit only
certain energies (wavelengths) of light.
•In complicated molecules or “solids” many
orbits and transitions are allowed
•Can
use energy levels to
“fingerprint” elements and estimate temperatures.
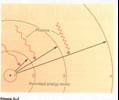
From our text: Horizons, by Seeds